Introduction
- Definition and Overview:
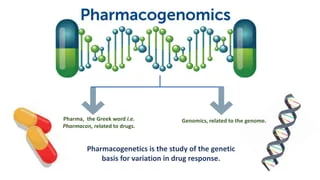
Pharmacogenomics is the study of how a person’s genetic makeup influences their response to drugs. It represents a significant step toward personalized medicine, where treatment is tailored based on an individual’s genetic profile, minimizing side effects and improving efficacy. - Importance of Pharmacogenomics in Healthcare:
Traditional “one-size-fits-all” drug treatments can lead to varied responses among patients due to genetic differences. Pharmacogenomics seeks to address this variability, ensuring optimal therapeutic outcomes.
1. Historical Background of Pharmacogenomics
- Early Discoveries:
The connection between genetics and drug response was first noticed in the mid-20th century. Research in pharmacogenetics began when scientists observed that individuals metabolize drugs differently. - Landmark Studies:
The identification of genetic polymorphisms (such as in enzymes like CYP2D6) marked significant progress, revealing why some individuals experience adverse effects from standard drug dosages. - The Human Genome Project and Its Impact:
Completed in 2003, the Human Genome Project provided an essential foundation for pharmacogenomics by mapping all human genes, paving the way for precision medicine.
2. Core Principles of Pharmacogenomics
- Genetic Variation and Drug Response:
Genetic polymorphisms in drug-metabolizing enzymes, drug transporters, and drug targets can significantly influence pharmacokinetics and pharmacodynamics. - Key Genes Involved:
- CYP450 enzyme family (e.g., CYP2D6, CYP3A4): Impacts metabolism of many drugs.
- UGT1A1: Involved in the metabolism of irinotecan, a chemotherapeutic agent.
- SLCO1B1: Impacts the metabolism of statins.
- Types of Genetic Variations:
- Single Nucleotide Polymorphisms (SNPs): The most common type of genetic variation that can affect drug metabolism.
- Copy Number Variations: Differences in the number of copies of a gene can impact drug response.
- Gene-Gene Interactions: Multiple genes often work together to influence how drugs are processed in the body.
3. Applications of Pharmacogenomics in Clinical Practice
- Cancer Treatment:
Pharmacogenomics has had a transformative impact on oncology. Treatments like trastuzumab for HER2-positive breast cancer and EGFR inhibitors in non-small-cell lung cancer are guided by genetic testing. - Cardiovascular Diseases:
Genetic variations in enzymes like CYP2C19 can affect the response to antiplatelet drugs like clopidogrel, necessitating adjustments in treatment protocols. - Psychiatry:
Pharmacogenomics is being applied to customize treatment for mental health conditions. Genetic testing can guide the use of antidepressants and antipsychotic medications, reducing the trial-and-error approach. - Infectious Diseases:
In HIV treatment, pharmacogenomic testing helps avoid hypersensitivity reactions (e.g., HLA-B*5701 testing before prescribing abacavir). - Pain Management:
Genetic factors influencing opioid metabolism (e.g., variations in CYP2D6) are used to prevent opioid toxicity or under-treatment of pain.
4. Key Pharmacogenomic Testing Technologies
- Next-Generation Sequencing (NGS):
NGS allows for the comprehensive analysis of multiple genes simultaneously, identifying variations that could affect drug metabolism. - Microarray Technology:
Microarrays enable the analysis of thousands of genetic variations at once, making it possible to identify SNPs relevant to pharmacogenomics. - Polymerase Chain Reaction (PCR):
PCR is widely used to detect specific genetic polymorphisms, making it a cornerstone of pharmacogenetic testing. - CRISPR and Gene Editing:
While not yet widely applied in clinical pharmacogenomics, CRISPR technology holds promise for modifying genes to optimize drug responses.
5. Ethical, Legal, and Social Implications (ELSI) of Pharmacogenomics
- Privacy Concerns:
Genetic information is sensitive, and there are concerns about data breaches or misuse by insurers or employers. - Informed Consent:
Patients must fully understand the implications of pharmacogenomic testing before undergoing genetic screening, which can be a challenge given the complexity of the science. - Equity in Access:
There is a risk that pharmacogenomics could exacerbate healthcare disparities, as testing may not be equally available to all populations. - Intellectual Property Issues:
The patenting of genetic discoveries has raised ethical concerns about accessibility and affordability of pharmacogenomic tests and treatments.
6. Challenges and Limitations of Pharmacogenomics
- Complexity of Genetic Influence on Drug Response:
While pharmacogenomics offers promise, predicting drug response is complex and involves multiple genes, environmental factors, and lifestyle influences. - Clinical Implementation:
Despite growing evidence, pharmacogenomics is not yet widely adopted in clinical practice due to cost, lack of provider education, and variability in test results. - Cost Considerations:
The high cost of genetic testing and lack of reimbursement by insurance companies are significant barriers to widespread adoption. - Regulatory and Standardization Issues:
The regulatory environment for pharmacogenomic tests is still evolving, with concerns about the validity and utility of certain tests.
7. The Future of Pharmacogenomics
- Integration with AI and Big Data:
Artificial Intelligence (AI) and machine learning algorithms are being developed to analyze vast amounts of genetic data, improving the predictive power of pharmacogenomic tests. - Pharmacogenomics in Rare Diseases:
There is increasing interest in using pharmacogenomics to develop treatments for rare genetic conditions, where conventional drugs often fail. - Expansion of Direct-to-Consumer Genetic Testing:
Companies like 23andMe and others are beginning to offer pharmacogenomic information directly to consumers, although the clinical utility of such data is still debated. - Pharmacogenomics in Preventive Medicine:
Looking ahead, pharmacogenomics could play a role in preventive care, allowing individuals to take proactive measures to avoid adverse drug reactions before they occur.





1 thought on “Pharmacogenomics: The Future of Personalized Medicine”
I love how you emphasize the importance of holistic health. It’s crucial to address both mind and body